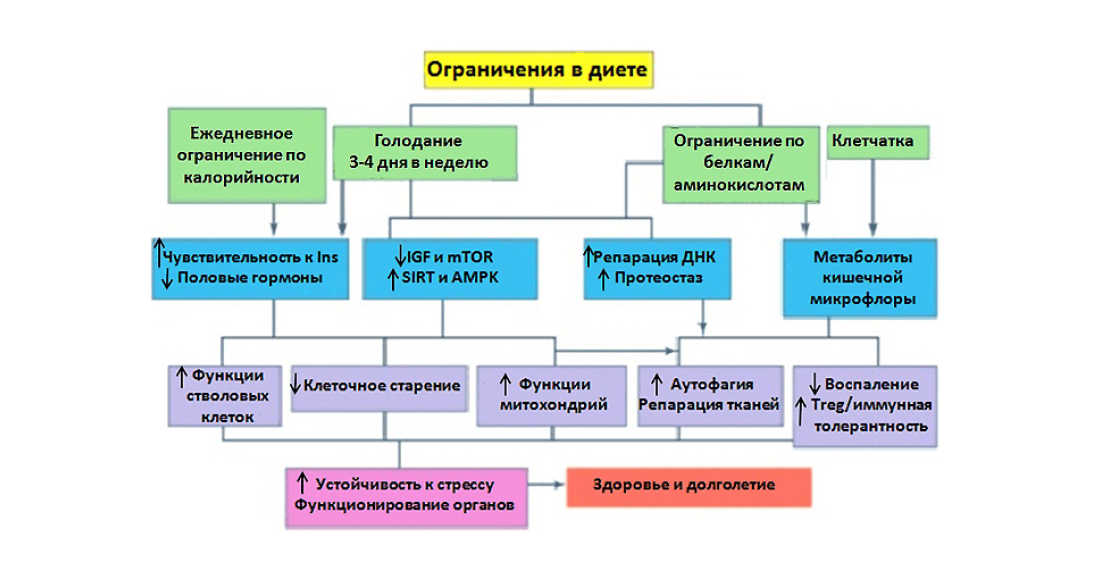
Effects of calorie and protein restriction in the diet on cellular and organismal physiology.
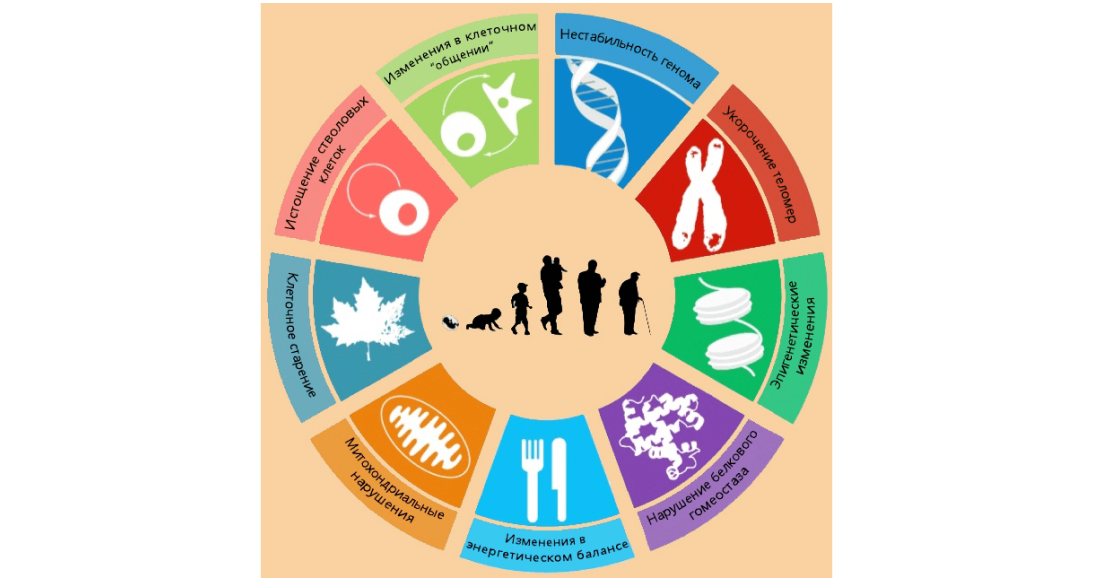
Loss of protein homeostasis
Protein homeostasis in the cell is maintained by two differently directed processes: mechanisms for the correct assembly of proteins (and their subsequent stabilisation) and mechanisms for the degradation of proteins with disturbed structure (proteolysis). If these processes fail, proteins aggregate, which leads to the development of neurodegenerative diseases
[15].
Heat shock proteins (HSPs) restore the disturbed structure of proteins, and under stress (thermal or chemical) the level of HSPs in the cell increases (Pic. 5). Stress-induced BTSH synthesis decreases significantly with age
[16], and this affects life span. SIRT1 protein in mammalian cells initiates BTSH synthesis
[17], and SIRT1 activity is increased by
resveratrol found in cranberries and grapes.
The efficiency of the two main proteolytic systems, autophagosomal (or lysosomal) and proteasomal, also decreases with age. Activation of autophagosomes slows cellular senescence and prolongs life in a number of model organisms. Spermidine, found in mushrooms, whole grains and legumes, triggers autophagy processes and its supplementation promotes longevity in worms, flies and mice
[18],
[19].
Disruption of protein homeostasis and enhancement of pro-inflammatory processes provoke
glycation end products (non-enzymatic reaction of carbohydrate attachment to amino acids in protein). High level of
advanced glycolation end products (AGEs) in tissues causes oxidative stress and inflammatory processes, as AGEs bind to cell surface receptors, trigger the inflammatory NF-kB-signalling pathway, as well as change the structure and functions of proteins
[20],
[21]. The findings show that reducing the amount of AGEs in food slows the development of chronic disease and aging in animals and apparently in humans. Vegetables, fruits, cereals, legumes, milk and bread contain low amounts of AGEs, while hard cheeses, beef, pork and poultry contain high amounts of AGEs
[21].
Genome stability
Accumulated damage in DNA during life is one of the basic signs of aging. Genome integrity and stability are constantly threatened by both external (chemical and biological agents) and internal factors (DNA doubling errors, reactive oxygen species). Genetic damage can affect and disrupt important biochemical pathways in the cell, which is particularly critical in the case of stem cells. In maintaining genomic stability, diet plays a more significant role than previously thought. B vitamins (B3, B9, B12), zinc and magnesium are essential for normal DNA synthesis, methylation and error correction, so even a slight deficiency of these substances in the organism affects genomic stability and leads to an increase in the level of spontaneous chromosomal damage
[22]. However, current norms for vitamins and minerals are set to prevent deficiencies rather than to minimise damage in DNA
[22]. The intake of these vitamins is particularly important for defects in their absorption/metabolism, which are commonly seen in old age. Therefore, after the age of fifty, consumption of foods with increased levels of B12 and B9 is recommended
[22]. People following a
vegan diet also need special vitamin supplements as B12 is only found in animal products.
Telomere length
Telomeres are the end sections of chromosomes whose length shortens with each cell division. Telomeres are bound to a multi-protein complex called shelterin, which prevents them from sticking together. Shelterin prevents the DNA from being accessed by repair systems that would recognise the ends of chromosomes as breaks in the DNA and join them together. Because of the limited repair, the damage that has occurred at telomeres is relatively persistent and can induce cell division arrest and/or cell apoptosis
[23],
[24]. Vitamins B3 and B9 are required to prevent damage and maintain normal telomere length
[25]. The consumption of omega-3-polyunsaturated fatty acids positively correlates with telomere length
[25],
[26].
Epigenetic changes
During life, epigenetic changes occur in the cells of our organism, which affect DNA methylation, histone modifications and rearrangement of chromatin structure (Pic. 6)
[7]. This leads to impaired DNA repair and increased chromosomal instability. However, unlike mutations, epigenetic processes are reversible: the activity of enzymes involved in the creation and maintenance of epigenetic marks can be regulated. With the help of changes in histone modifications, scientists have increased the lifespan of progeroid mice (mice with accelerated aging)
[27]and restored cognitive abilities in old mice
[28]. Many substances have been found in fruits, vegetables and greens that influence the activity of enzymes involved in epigenetic remodelling
[29].
Genistein isolated from soya induces the establishment of certain histone modifications (H3K27 and H3K9 methylation), the level of which decreases with age
[29]. And one of the effects of resveratrol contained in cranberries, blueberries, grapes and red wine is an increase in the activity of the SIRT1 protein involved in histone modification.
Sirtuin proteins, which belong to the family of NAD-dependent deacetylases (i.e., they remove the acetyl tag from histones), have been widely studied as potential anti-aging factors. Increased expression of SIRT1 in mammals improves health outcomes in senescence (life expectancy is not increased)
[32]. In addition, strong evidence for SIRT6 has been obtained for its activity on longevity in mammals
[33]. And recent experiments have shown that free fatty acids (oleic, linolenic, and myristic acids) in physiological concentrations increase SIRT6 activity
[34].
Mitochondrial disorders
Mitochondria are the main energy stations of the cell; they oxidise incoming nutrients, converting them into energy in the form of ATP
[35]. During the oxidation of substances in mitochondria, oxygen radicals (
reactive oxygen species – AOS) are inevitably formed, which damage cellular structures
[36],
[37]. Previously, it was believed that mitochondrial damage contributes to aging precisely because of increased production of AFCs. However, recent data cast doubt on this hypothesis. Disturbances in mitochondria, regardless of the level of AFC, lead to cell apoptosis and increased inflammatory responses
[38]. Mitochondrial dysfunction with age occurs for several reasons. Firstly, the formation of new mitochondria (mitochondriogenesis) decreases due to damage in DNA and telomere shortening. In addition, mutations accumulate in mitochondrial DNA due to the AFC-rich environment and limited efficiency of repair systems in mitochondria (compared to the nucleus)
[7]. The same SIRT1 protein activates mitochondriogenesis, increases the antioxidant defence of the cell
[39] and promotes the removal of damaged mitochondria through the process of autophagy
[40]. There is another way to improve mitochondrial function. It is known that mild toxins provoke defence reactions in the cell, making it less susceptible to various unfavourable factors. In response to weak mitochondrial poisons, which include resveratrol
[41] (contained in grapes, blueberries, and cranberries), mitochondrial control genes are activated in the cell to ensure mitochondrial integrity and functionality
[42].
Cellular aging, changes in intercellular communication and stem cell depletion
Stem cell depletion and changes in intercellular communication are the main ‘culprits’ of the aging phenotype: brittle bones, reduced muscle mass, weakening of the immune system, and so on. One of the reasons for the development of these signs of aging is cellular senescence or division arrest. Cellular senescence is induced by telomere shortening, DNA damage or abnormalities in cell division signals. At the same time, senescent cells secrete specific molecules (pro-inflammatory cytokines and metalloproteinases) that accelerate the aging of surrounding cells as well as initiate inflammatory reactions
[43],
[44]. Due to stem cell depletion, the level of immune cells falls and defects in their activation are observed
[45]. In sum, this leads to weakening of immune defence, enhancement of pro-inflammatory reactions and triggering of NF-kB inflammatory signalling pathway.
But some functions of the immune system can be restored by nutrition. For example, increased doses of vitamin E can enhance T-cell function in the elderly; dietary intake of the amino acid tryptophan and fibre has a beneficial effect on the structure and function of the intestinal microflora and, consequently, on its secretion of factors that regulate multiple inflammatory and metabolic pathways (Pic. 7)
[13]. The intestinal microflora produces specific molecules that aid the division and differentiation of regulatory T cells (and regulatory T cells play an important role in controlling inflammation and autoimmune responses)
[46],
[47]. Population analyses have found strong correlations between diet and microflora composition, and between microflora composition and morbidity as well as inflammation levels in the elderly
[48]. Manipulation of the composition of the intestinal microflora appears to be another effective way to increase longevity and improve well-being in old age
[48],
[49].